©1996-2002 All Rights Reserved. Online Journal of Veterinary Research. You may not store these pages in any form except for your own personal use. All other usage or distribution is illegal under international copyright treaties. Permission to use any of these pages in any other way besides the before mentioned must be gained in writing from the publisher. This article is exclusively copyrighted in its entirety to OJVR publications. This article may be copied once but may not be, reproduced or re-transmitted without the express permission of the editors.
Online Journal of Veterinary Research
Volume 1:7-28, 2002 .
In Koo Hwang (DVM, MS), Heungshik S Lee (DVM, PhD), Young Sam Nam (DVM, BS), Choong Hyun Lee (DVM, MS), Yeo Sung Yoon (DVM, PhD), Tae-Cheon Kang* (DVM, PhD), Moo Ho Won* (DVM, PhD), In Se Lee+ (DVM, PhD),
Department of
Anatomy,
College of Veterinary Medicine and School of Agricultural
Biotechnology,
Seoul National University, Suwon, Kyunggi-Do, 441-744, South
Korea.*Department
of Anatomy, College of Medicine, Hallym University. Chunchon,
Kangwon-Do,
200-702, South Korea.+Corresponding Author, Dr. In Se Lee, Department
of
Anatomy, College of Veterinary Medicine and School of Agricultural
Biotechnology,
Seoul National University, Suwon, Kyunggi-Do, 441-744, South Korea,
TEL:
+82-31-290-2743, FAX: +82-31-290-2717, E-mail: inselee@snu.ac.kr
Immunohistochemical and double immunofluorescent was used to identify the localization of calbindin D-28k (CB), calretinin (CR), parvalbumin (PA), substance P (SP), calcitonin gene-related peptide (CGRP) and galanin (GAL) in the goat small intestine. Localization of CB with SP, CGRP or GAL in the myenteric and submucosal plexuses suggests that CB may serve a neuromodulatory role for SP-, CGRP-, and GAL-immunoreactive neurons in intestinal wall motility. Distributions of CB, CR and SP of the goat small intestine differ from guinea-pig and the pig. This difference may be relevant to morphological characteristics.
INTRODUCTION
The enteric nervous system (ENS) is one of the three portions of the autonomic nervous system and can perform its function independently of the central nervous system. It consists of local nerve networks embedded in the gut wall and is subdivided into two ganglionated plexuses, i.e., the submucosal and myenteric plexuses. In large animals, however, a further division of the submucous plexus may be present, i.e., an inner submucosal nerve network, located close to the abluminal side of the laminal muscularis mucosae, and an outer one, lying adjacent to the luminal side of the circular smooth muscle layer. In the human small intestine, even a third ganglionated plexus, the so-called intermediate ganglionic plexus, can be distinguished (Timmermans et al, 1997).
The ENS mediates complex reflex activities involving intestinal motility, mucosal transportation and blood flow. These functions are achieved by complex interactions within the enteric networks, which consist of sensory neurons, interneurons, and motor neurons, each of which is likely to synthesize a diverse set of neurotransmitters (Hens et al., 2000).
Recent immunohistochemical investigations have revealed that ranges of neurotransmitters or neuromodulators are contained within the enteric neurons. Thus, the concept of neurochemical coding is considered an important aspect of the understanding of enteric microcircuits and their functions. Moreover, the colocalization of these chemical substances has contributed to identifying the roles of the enteric neurons. In addition, a variety of biologically active peptides have been recognized in nerves of the gut wall of the intestine, and it has also been reported that substance P (SP), calcitonin gene-related peptide (CGRP) and galanin (GAL) mainly modulate the motility and/or the sensation of the gut in mammalians.
Calbindin D-28k (CB), calretinin (CR) and parvalbumin (PA) are members of the EF-hand calcium binding protein family, and specifically bind intracellular free calcium in the micromolar range. CB may act either as a calcium transporter or as an intracellular calcium buffer, promoting or restricting calcium-dependent events in the cellular metabolism (Persechini, 1989). Moreover, these calcium-binding proteins may modulate the release or action of SP, CGRP and GAL (Resibois et al., 1988).
Significant species
differences
may exist in terms of the anatomical structure and the range of
neurotransmitters
or neuromodulators, even between closely related species. A number of
studies
have been performed upon the peptide-containing neurons of a wide
variety
of species, including man, the guinea-pig, the pig and the horse (Hens
et al., 2000; Li and Furness, 1998; Bagnol
et al., 1997; Costa et al., 1996; Dhatt
et al., 1994; Song et al., 1994; Kirchgessner
and Gershon, 1991; Song et al., 1991; Dahlstrand
et al., 1988; Resibois et al., 1988; Furness
and Costa, 1980). However, few studies have been undertaken on the
neurons of the goat enteric nervous system. The aim of this study was
to
identify the localization of CB, CR, PA, SP, CGRP and GAL in the goat
small
intestine. The present study also attempts to elucidate the anatomical
differences of ENS between the ruminant and the guinea-pig, dog, horse
and pig.
Twelve goats (Capra hircus, 10-16months, 15-20 kg B.W.) were used in this study. Goats were obtained from Samtaco Animal Center (Korea). The animals were anesthetized with ketamine-xylazine mixture and perfused via the common carotid artery with 3 L of 0.85 % normal saline. Small intestines were opened along the mesenteric attachment, and the tissues were cut out from the intestinal wall at three portions – the sigmoid flexure of the duodenum, and middle parts of the jejunum and ileum. The tissues were stretched and pinned flat on pieces of balsa wood. The tissues were then fixed in 4% paraformaldehyde in phosphate buffered saline (PBS, pH 7.4) with mucosal surface facing up, and cryoprotected in 30% sucrose in PBS. Serial sections of 14m were cut using Cryostat (Reichert-Jung, Germany) and mounted on gelatin-coated slides. The sections were stored at –70 ? for 1-3 weeks before processing for immunohistochemistry and double immunofluorescence.
The sections were treated with 3% H2O2 in methanol for 15 minutes followed by 5% normal goat serum (Dako, USA) for 30 minutes to reduce nonspecific background staining. Some sections were then incubated in primary antibodies (Table 1) containing 0.3% triton X-100 and 2% normal goat serum overnight at room temperature. After washing three times for 10 minutes with PBS, sections were sequentially incubated, in goat anti-rabbit IgG (Vector, USA) and streptavidin (Vector, USA), diluted 1:200 in the same solution as primary antisera. Between the sequential incubations, the tissues were washed with PBS three times for 10 minutes each. The sections were visualized with DAB (3,3’-diaminobenzidine) in 0.1M Tris buffer, and mounted on gelatin-coated slides. Immunoreactions were observed under an Axioplan microscope (Carl Zeiss, Germany).
For co-localization
studies,
the tissues were incubated with mixtures of mouse monoclonal CB and
rabbit
polyclonal CR, SP, CGRP, GAL antibodies. The antibody-antigen complexes
were visualized using secondary antibodies conjugated with differenent
fluorochromes (Table 1), i.e.,
indocarbocyanine
(Cy3)-conjugated anti-mouse IgG for CB, and fluorescein isothiocyanate
(FITC)-conjugated anti-rabbit Igg for CR, SP, CGRP and GAL.
Flourescence
labeling was examined under an Axioplan microscope (Carl Zeiss,
Germany),
using appropriate filters.
TABLE 1: ANTIBODY USED FOR DOUBLE IMMUNOFLUORESCENT METHODS
| Antibodies | Hosts | Dilution | Sources |
| CB | Rabbit | 1:10000 | Chemicon, USA |
| Mouse | 1:500 | Peninsula, USA | |
| CR | Rabbit | 1:5000 | Chemicon, USA |
| PA | Mouse | 1:5000 | Chemicon, USA |
| SP | Rabbit | 1:10000 | Peninsula, USA |
| CGRP | Rabbit | 1:10000 | Peninsula, USA |
| GAL | Rabbit | 1:20000 | Chemicon, USA |
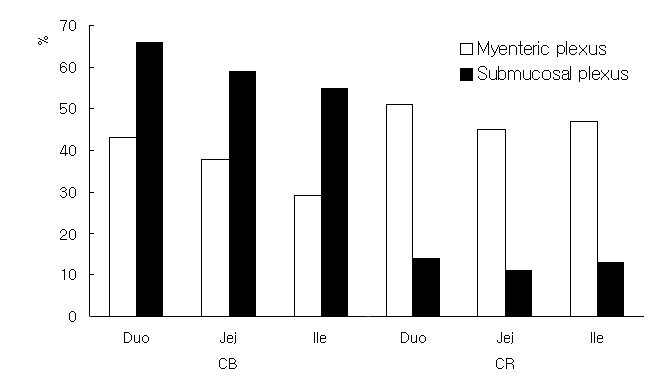
CB-, CR-, SP-, CGRP-, and GAL-like immunoreactivities (IR) were demonstrated in both nerve cell bodies and fibers of the neurons of the myenteric and submucosal plexuses of the goat small intestine. The complete histological images are very large (6mb each) and sections are shown herein in Figures 3-6. If you wish to view the complete image click on complte image at the bottom of each figure, respectively.
SP-IR was observed
in
the myenteric plexus as well as in the submucosal plexus, but the
number
of SP-immunoreactive neurons was more numerous in the submucosal plexus
than in the myenteric plexus (Figure 1). CB-IR was prominent in both
plexuses,
but CB-immunoreactive neurons were more abundant in the submucosal
plexus
than in the myenteric plexus. CR-IR cells and their immunoreactivities
were more abundant in the myenteric plexus than in the submucosal
plexus
and PA-IR was observed only in the epithelia of villi and the
intestinal
crypt but not in the plexuses. The CB-IR of the submucosal plexus
gradually
decreased on progressing from the duodenum to the ileum in both
plexuses
and the number of CR positive neurons was similar in the three portions
of the small intestine.

Figure 1. Ratio of immunoreactive cells for calcium binding proteins in neurons of the myenteric and submucosal plexuses in the Korean native goat small intestine.CGRP-IR was found in both the submucosal and myenteric plexuses. However, its immunoreactivity was relatively weaker and the number of immunoreactive neurons was fewer than those for CB, CR, SP and GAL. GAL-IR was observed primarily on nerve fibers in the submucosal and myenteric plexuses, and the immunoreactivity was particularly strong in the jejunum of both plexuses. Some CR-, SP-, and GAL-immunoreactive nerve fibers arose from the myenteric plexus and ran across the inner muscle layer toward the mucosa.
In the myenteric and submucosal plexuses, most of the CB immunoreactive neurons were immunoreactive for SP. The colocalization of CB with CR, CGRP and GAL was also confirmed in both plexuses, but the number of these double-immunoreactive cells was much fewer than that of cells doubly immunoreactive for CB and SP. SP containing neurons gradually decreased in number on progressing from duodenum to the ileum in the submucosal plexus, but increased in the myenteric plexus as shown in Figure 2.
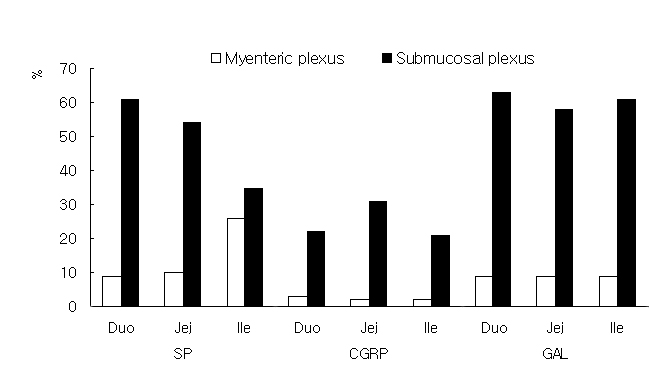
Figure 2. Ratio of immunoreactive cells for substance P, CGRP and galanin in neurons of the myenteric and submucosal plexuses in the Korean native goat small intestine.
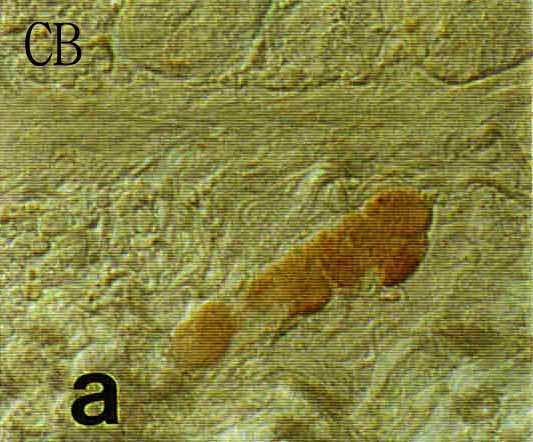
Figure 3. One
section
of micrographs of longitudinal sections of the submucosal (upper
plates)
and myenteric (lower plates) plexuses in Korean native goat small
intestine.
The sections were immunostained with one of anti-calbindin D-28k (CB),
calretinin (CR), parvalbumin. a, b : duodenum c, d :
jejunum
e, f : ileum (PA a, b only a in Fig. 3). To
view
the complete image (6 megabytes) click
here. This may take several minutes
Fig. 4. Micrographs
of
longitudinal sections of the submucosal (upper plates) and myenteric
(lower
plates) plexuses in Korean native goat small intestine. ), substance P
(SP), calcitonin gene-related peptide (CGRP) and galanin (GAL), and
visualized
by DAB (×200). a, b : duodenum c, d : jejunum
e,
f : ileum. To view the complete image (6
megabytes)
click
here. This may take several minutes.
Fig. 5. Micrographs of double-immunostained sections of the submucosal (upper plates) and myenteric (lower plates) plexuses of the duodenum Korean native goat small intestine (duodenum). The sections were double-immunoreacted with the mixture of anti-mouse CB and one of anti-rabbit CR, SP and then fluoresced by Cy3-conjugated IgG (CB; left plates) and FITC-conjugated IgG (CR and SP; right plates) (×200). a, b : submucosal plexus c, d : myenteric plexus. To view the complete image (6 megabytes) click here. This may take several minutes.
Fig. 6. Micrographs
of
double-immunostained sections of the submucosal (upper plates) and
myenteric
(lower plates) plexuses of the duodenum Korean native goat small
intestine
(duodenum). The sections were double-immunoreacted with the mixture of
anti-mouse CB and one of anti-rabbit CGRP and GAL, and then fluoresced
by Cy3-conjugated IgG (CB; left plates) and FITC-conjugated IgG (CGRP
and
GAL,; right plates) (×200). a, b : submucosal plexus
c, d : myenteric plexus. To view the complete
image (6 megabytes) click
here. This may take several minutes.
DISCUSSION
Recently, many studies have provided data on the chemical coding within the myenteric and submucosal plexuses in various animals (Rasmussen et al., 2001;Hens et al., 2000). We report for the first time on the results of a similar approach on the myenteric and submucosal neurons in the goat small intestine. Based on their neurochemical coding, we identified the distributions of some neuropeptides and calcium binding proteins. We found four subpopulations of co-localization, i.e., CB/CR, CB/SP, CB/CGRP and CB/GAL, which is a further indication of the multiple transmitter mechanisms in enteric nerves in general.
Our chemical coding evaluation is based on the relative counts of antigen-immunoreactive cell bodies. Neuron specific enolase (NSE)-IR was used as a nonspecific marker for cell bodies, because NSE antibodies have been previously used as a tool for locating enteric ganglia and nerve fibers in the intestine (Vento et al., 2001).
The distribution of
CB,
CR, PA, SP, CGRP and GAL immunoreactivity has been demonstrated
immunohistochemically
in neurons and their processes in the goat small intestine. It is known
that there are several histologically distinct intrinsic neuronal types
in the ENS. These include excitatory and inhibitory motor neurons to
the
muscle, vasomotor neurons, secretomotor neurons, interneurons and
sensory
neurons. Many investigators have tried to relate these physiological
functions
to individual cytoarchitecturally defined enteric neurons (Furness
et al., 1987; Furness et al., 1988; Gabella,
1971). Classically, Dogiel classified enteric neurons into three
types.
He suggested that type-I cells were motor neurons as their axons
extended
towards muscle. Moreover, because many of the CR, SP and GAL-positive
neurons
were Dogiel type-I neurons and their fibers were dense in both the
muscular
layer and the myenteric plexuses, it was suggested that CR, SP and
GAL-immunoreactive
neurons in the myenteric plexus may be associated with the control of
the
gut motility.
Although the patterns
of staining in the submucosal and myenteric plexuses were quite
different
in some neuronal subpopulations compared with other animals, there are
few striking similarities.
In the goat, CB-immunoreactive neurons were more abundant in the submucosal plexus than in the myenteric plexus. This result is contradictory to that found in the rat, guinea pig, human and monkey (Clerc et al., 1998; Goodman and Iversen, 1986; Walters et al., 1993; Resibois et al., 1988). In contrast to CB, CR-immunoreactive neurons were more abundant in the myenteric plexus, as found in the guinea pig. However, though this result is similar to that of the guinea-pig, it does not agree with those of the human (Clerc et al., 1998; Goodman and Iversen, 1986).
CB-containing neurons have been reported to be present in the intestinal myenteric plexus of various species, including, the guinea pig, rat, pig, domestic fowl and the human (Furness et al., 1988; Scheuermann et al., 1991; Walters et al., 1993; Lunam, 1993). In the guinea pig small intestine, about one-third of all myenteric nerve cells have been reported to be immunoreactive for CB and believed to be Dogiel type II neurons (Furness et al., 1988). These cells have the electrophysiological characteristics of AH/type 2 neurons (Iyer et al., 1988), and may be considered to be intrinsic sensory neurons (Furness et al., 1988). In the goat, CB-IR cells also have the Dogiel type II morphology. Because CB-positive Dogiel type II neurons actually perform a sensory function in the small intestine, neurons doubly immunoreactive for CB and SP or other peptides are thought to have a sensory function that may modulate the sensation of the gut lumen. The results that CB and SP-IR neurons are more abundant in the duodenum than in the jejunum and ileum indicates that they have more important roles in the duodenum during absorption or digestion. On the other hand, CR-immunoreactive neurons with the morphological characteristics of Dogiel type I cells may modulate the motility of muscles in both the inner and outer layers.
Although the functions of SP-IR neurons have not been elucidated, the abundance of SP-IR varicose fibers within both muscle layers suggests that most of the SP cells might function as motor neurons. SP-immunoreacted neurons colabeled with CR may be motor neurons to the longitudinal muscle, as has been suggested for the small intestine (Brooks et al., 1992).
In a variety of species, SP has been shown to be the major excitatory neurotransmitters of motor neurons in the gastrointestinal tract (Holzer and Lippe, 1984; Maggi et al., 1994). However, a significant difference in the density of SP was noticed among the different regions of the digestive tract. In contrast to small animals in which SP-IR neurons were found in the submucosal and myenteric plexuses, SP-IR is absent or very weak in the plexuses of large animals. Pearson (1994) reported that in the horse, SP immunoreactivity was not observed in any of the neurons of the submucosal plexus and that only weak SP-immunoreactivity was seen in the myenteric plexus. In the pig, Hens et al (2000) reported SP-immunoreactive neurons and fibers in both plexuses, while Schmidt et al (1991) reported that only SP-IR fibers were evident in the myenteric plexus.
A possible
explanation
for this discrepancy might be that ganglionic organization is different
between small and large animals. The small intestines of large animals
such as the horse, pig and dog are relatively thick. In these animals,
ENS is composed of three ganglia, whereas that of small animals such as
the rat, mouse and guinea-pig is composed of two. The submucosal
plexuses
of large animals are subdivided into an inner submucosal plexus, which
is located close to the abluminal side of the lamina musclularis
mucosa,
and an outer submucosal plexus lying adjacent to the luminal side of
the
circular muscle layer (Timmermann et al., 1997).
In
these animals, therefore, the SP-IR fibers of the inner circular muscle
layer originated from the outer submucosal plexus rather than the
myenteric
plexus. Although the small intestine of the goat is composed of three
ganglia
(data not shown), the distribution parttern of SP-IR neurons is quite
unlike
that of the horse and the pig. This result indicates that the origins
of
the fibers located in the inner circular muscle may be the inner
submucosal
and myenteric plexuses. But the retrograde tracing studies remain to be
elucidated.
The occurrence,
function
and frequency of CGRP fibers and neurons in the myenteric and
submucosal
ganglia have been reported to differ markedly from one species to
another.
Whereas the guinea-pig was more or less devoid of myenteric CGRP fibers
in the intestine (Goodman and Iversen, 1986),
such
fibers and neurons were numerous in the human (Timmermann
et al, 1992), pig (Hens et al., 2000) and
dog
(Sternini et al., 1992). This difference may be
the result of the morphological characteristics of the small intestine.
In this study, the immunoreactivities of GAL were denser in the jejunum and ileum than in the duodenum. These results may reflect the fact that the major roles of the jejunum and ileum are peristaltic movement and rhythmic segmentation, and that these are more potent in the goat than in the other animals, because the role of GAL in the ENS is known to be excitatory (Korolkiewicz et al., 2000). The localization of CB, CR, SP, CGRP and GAL in the enteric neurons of the intestinal wall showed that these substances may have an important role in intestinal movement. The colocalization of CB with SP, CGRP or GAL in the myenteric and submucosal plexuses provides anatomical support that CB may serve a neuromodulatory role for SP-, CGRP-, and GAL-immunoreactive neurons in terms of intestinal wall motility. Distributions of CB, CR and SP in the goat small intestine differed from those of the guinea pig and pig. This suggests that the mechanisms and neurotransmitters involving enteric circuits in the goat differ from those in other animals. Moreover, this difference may be relevant to the morphological characteristics of digestive tracts.
In conclusion, this
study
demonstrates that some striking species differences may occur in the
peptide
and calcium binding protein distributions of the goat and the more
extensively
studied animals, like guinea-pig.
Bagnol
D, Henry M, Cupo A, Jule Y (1997), Distribution of enkephalin-like
immunoreactivity
in the cat digestive tract., J Auton Nerv Syst 64 p 1-11.
Brookes
SJH, Song ZM, Steele PA, Costa M (1992), Identification of motor
neurons
to the longitudinal muscle of the guinea pig ileum., Gastroenterology
103
p 961-973.
Clerc
N, Furness JB, Bornstein JC, Kunze WAA (1998), Morphological and
immunohistochemical
identification of neurons and their targets in the guinea-pig
duodenum.,
Neuroscience 86 p 679-694.
Costa
M, Brookes SJ, Steele PA, Gibbins I, Burcher E, Kandiah CJ (1996),
Neurochemical
classification of myenteric neurons in the guinea-pig ileum.,
Neuroscience
75 p 949-967.
Dahlstrand
C, Bjeck S, Edin R, Dahlstram A, Ahlman H (1988), Substance P in the
control
of extrahepatic biliary motility in the cat., Regul Pept 20, p 11-24.
Dhatt
N, Buchan, AM (1994), Colocalization of neuropeptides with calbindin
D28K
and NADPH diaphorase in the enteric nerve plexuses of normal human
ileum.,
Gastroenterology 107 p 680-690.
Furness
JB, Costa M (1980), Types of nerves in the enteric nervous system.,
Neuroscience
5 p 1-20.
Furness
JB, Costa M, Rokaeus A, McDonald TJ, Brooks B (1987),
Galanin-immunoreactive
neurons in the guinea-pig small intestine: their projections and
relationships
to other enteric neurons., Cell Tissue Res 250 p 607-615.
Furness
JB, Keast JR, Pompolo S, Bornstein JC, Costa M, Emson PC, Lawson DE
(1988)
Immunohistochemical evidence for the presence of calcium-binding
proteins
in enteric neurons. Cell Tissue Res 252 p 79-87.
Gabella
G (1971), Neuron size and number in the myenteric plexus of the newborn
and adult rat., J Anat 109 p 81- 95.
Goodman
EC, Iversen LL (1986) Calcitonin gene-related peptide: novel
neuropeptide.,
Life Sci 38 p 2169-2178.
Hens
J, Schrodl F, Brehmer A, Adriaensen D, Neuhuber W, Scheuermann DW,
Schemann
M, Timmermans JP (2000) Mucosal projections of enteric neurons in the
porcine
small intestine., J Comp Neurol 421 p 429-436.
Holzer
P, Lippe IT (1984) Substance P can contract the longitudinal muscle of
the guinea-pig small intestine by releasing intracellular calcium., Br
J Pharmacol 82 p 259-267.
Iyer
V, Bornstein JC, Costa M, Furness JB, Takahashi Y, Iwanaga T (1988)
Electrophysiology
of guinea-pig myenteric neurons correlated with immunoreactivity for
calcium
binding proteins., J Auton Nerv Syst 22 p 141-150.
Kirchgessner
AL, Gershon MD (1991), Innervation and regulation of the pancreas by
neurons
in the gut., Z GastroenterolVerh 26 p 230-233.
Korolkiewicz
RP, Konstanski Z, Rekowski P, Ruczynski J, Szyk A, Korolkiewicz KZ,
Petrusewicz
J (2000), Sources of activator Ca2+ for galanin-induced contractions of
rat gastric fundus, jejunum and colon., J Physiol Pharmacol 51 p
821-831.
Kulkarni-Narla
A, Beitz AJ, Brown DR (1999), Catecholaminergic, cholinergic and
peptidergic
innervation of gut-associated lymphoid tissue in porcine jejunum and
ileum.,
Cell Tissue Res 298 p 275-286.
Li ZS,
Furness JB (1998), Immunohistochemical localisation of cholinergic
markers
in putative intrinsic primary afferent neurons of the guinea-pig small
intestine., Cell Tiss Res 294 p 35-43.
Lunam
CA (1993), Calbindin, tyrosine hydroxylase and opioid-like
immunoreactivity
in the intestinal nerve of Remak of the domestic fowl., J Auton Nerv
Syst
44 p 189-196.
Maggi
CA, Patacchini R, Meini S, Quartara L, Sisto A, Potier E, Giuliani S,
Giachetti
S (1994), Comparison of tachykinin NK1 and NK2 receptors in the
circular
muscle of the guinea-pig ileum and proximal colon., Br J Pharmacol 112
p 150-160.
Pearson
GT (1994), Structural organization and neuropeptide distributions in
the
equine enteric nervous system: an immunohistochemical study using
whole-mount
preparations from the small intestine., Cell Tiss Res 276 p 523-534.
Persechini
A, Moncrief ND, Kretsinger RH (1989), The EF-hand family of
calcium-modulated
proteins., Trends in Neurosci 12 p 462-467.
Rasmussen
TN, Schmidt P, Poulsen SS, Holst JJ (2001), Localisation and neural
control
of the release of calcitonin gene-related peptide (CGRP) from the
isolated
perfused porcine ileum., Regul Pept 98 p 137-143.
Resibois
A, Vienne G, Pochet R (1988), Calbindin-D28K and the peptidergic
neuroendocrine
system in rat gut: an immunohistochemical study., Biol Cell 63 p 67-75.
Scheuemann
M, Schaaf C, Mader M (1995), Neurochemical coding of enteric neurons in
the guinea pig stomach., J Comp Neurol 353 p 161-178.
Schmidt
P, Poulsen SS, Rasmussen T, Bersani M, Holst JJ (1991), Substance P and
neurokinin A are condistributed and colocalized in the porcine
gastrointestinal
tract., Peptides 12 p 963-973.
Song
ZM, Brookes SJ, Costa M (1994), All calbindin-immunoreactive myenteric
neurons project to the mucosa of the guinea-pig small intestine.,
Neurosci
Lett 180 p 219-222.
Song
ZM, Brookes SJ, Costa M (1991), Identification of myenteric neurons
which
project to the mucosa of the guinea-pig small intestine., Neurosci Lett
129 p 294-298.
Sternini
C, de Giorgio R, Furness JB (1992), Calcitonin gene-related peptide
neurons
innervating the canine digestive system., Regul Pept 42 p 15-26.
Timmermans
J-P, Adriaensen D, Cornelissen W, Scheuermann DW (1997), Structural
organization
and neuropeptide distribution in the mammalian enteric nervous system,
with special attention to those components involved in mucosal
relexes.,
Comp Biochem Physiol [A] 118 p 331-340.
Timmermans,
JP, Scheuermann DW, Stach W, Adriaensen D, de Groodt-Lasseel MH (1992),
Functional morphology of the enteric nervous system with special
reference
to large mammals., Eur J Morphol 30 p 113-122.
Vento
P, Kiviluoto T, Keranen U, Jarvinen HJ, Kivilaakso E, Soinila S (2001),
Quantitative comparison of growth-associated protein-43 and substance P
in ulcerative colitis., J Histochem Cytochem 49 p 749-758.
Walters
JRF, Bishop AE, Facer P, Lawson DEM, Rogers JH, Polak JM (1993),
Calretinin
and calbindin-D28k immunoreactivity in the human gastrointestinal
tract.,
Gastroenterology 104 p 1381-1389.
Fig. 3. Micrographs of longitudinal sections of the submucosal (upper plates) and myenteric (lower plates) plexuses in Korean native goat small intestine. The sections were immunostained with one of anti-calbindin D-28k (CB), calretinin (CR), parvalbumin. a, b : duodenum c, d : jejunum e, f : ileum (PA a, b only a in Fig. 3).
Fig. 4. Micrographs of longitudinal sections of the submucosal (upper plates) and myenteric (lower plates) plexuses in Korean native goat small intestine. ), substance P (SP), calcitonin gene-related peptide (CGRP) and galanin (GAL), and visualized by DAB (×200). a, b : duodenum c, d : jejunum e, f : ileum.
Fig. 5. Micrographs of double-immunostained sections of the submucosal (upper plates) and myenteric (lower plates) plexuses of the duodenum Korean native goat small intestine (duodenum). The sections were double-immunoreacted with the mixture of anti-mouse CB and one of anti-rabbit CR, SP and then fluoresced by Cy3-conjugated IgG (CB; left plates) and FITC-conjugated IgG (CR and SP; right plates) (×200). a, b : submucosal plexus c, d : myenteric plexus.
Fig. 6. Micrographs of double-immunostained sections of the submucosal (upper plates) and myenteric (lower plates) plexuses of the duodenum Korean native goat small intestine (duodenum). The sections were double-immunoreacted with the mixture of anti-mouse CB and one of anti-rabbit CGRP and GAL, and then fluoresced by Cy3-conjugated IgG (CB; left plates) and FITC-conjugated IgG (CGRP and GAL,; right plates) (×200). a, b : submucosal plexus c, d : myenteric plexus.
©1996-2002 All Rights Reserved. Online Journal of Veterinary Research. You may not store these pages in any form except for your own personal use. All other usage or distribution is illegal under international copyright treaties. Permission to use any of these pages in any other way besides the before mentioned must be gained in writing from the publisher. This article is exclusively copyrighted in its entirety to OJVR publications. This article may be copied once but may not be, reproduced or re-transmitted without the express permission of the editors.