©1994-2003 All Rights Reserved. Online Journal of Veterinary Research. You may not store these pages in any form except for your own personal use. All other usage or distribution is illegal under international copyright treaties. Permission to use any of these pages in any other way besides the before mentioned must be gained in writing from the publisher. This article is exclusively copyrighted in its entirety to OJVR publications. This article may be copied once but may not be, reproduced or re-transmitted without the express permission of the editors.
OJVRTM
Online Journal of Veterinary Research©
Volume 7 : 99-105, 2003.
Evaluation of the efficacy of eformoterol on Exercise-induced Pulmonary Hemorrhage in training thoroughbred horses.
Ladaga GJB1,2, Pont Lezica F1, Ulloa F1, de Erausquin GA1,3, Ruzzante G1, Negrelli C1 del Carril R1
INCA Group1, Buenos Aires, Argentina; Laboratorio Fundación2, Buenos Aires, Argentina; and Washington University School of Medicine3, St Louis, Missouri. Address for correspondence: Gabriel A. de Erausquin, MD, PhD Washington University School of Medicine, 660 S. Euclid Ave, Campus Box 8134, St. Louis, MO 63110 e-mail: erausquing@neuro.wustl.edu
ABSTRACT
KEYWORDS: eformoterol, thoroughbred, exercise-induced pulmonary hemorrhage.
The etiology of EIPH is not fully understood, but a combination of respiratory, circulatory and mechanical factors have been invoked (Donaldson LL, 1991, Johnson et al, 1992). Pathological studies in horses with history of EIPH revealed that dorsocaudal lung fields were most affected, with thickened bronchioles and neovascularization suggestive of small airway disease and secondary proliferation of the bronchial circulation (O´Callaghan et al, 1987, Oikawa M, 1999). These anatomical changes are likely to be chronic, and due to multiple instances of EIPH. Experimental studies in excised lungs from rabbits, dogs and horses have shown that exaggerated transmural alveolar pressures can cause acute disruption of the alveolar epithelium and capillary endothelium leading to interstitial edema and intra-alveolar hemorrhage (Birks et al 1997). Such findings have led to the hypothesis that EIPH is due to a combination of mechanical stress caused by extremely negative intra-alveolar pressures during inspiration and elevated capillary and pulmonary arteriolar pressures (Birks et al 1997), with secondary inflammatory responses leading to neovascularization. Lastly, since EIPH is not seen in horses undergoing sustained exercise but is nearly universal in horses after strenuous maximal effort, it is reasonable to assume that mechanical factors are also involved.
Based on these pathophysiological data, we decided to assess the protective effect of the the b2 adrenergic receptor agonist eformoterol on EIPH in thoroughbred horses undergoing competitive training. Eformoterol appears to be a rational choice for treatment EIPH on several grounds (Anderson GP, 1993). First, eformoterol is a powerful bronchodilator, and inhibitor of bronchoconstriction stimulated by angiotensin II, adenosine, tachykines, and histamine (Hoffman and Lefkowitz 1996, Ochsner M, 1996, Nightingale et al 1999, Verleden et al 1993, Advenier et al 1992, Nightingale et al 1999). Second, it reduces tachikynin and histamine-induced increases in endothelial permeability and ensuing plasma exhudation, as well as tachykinin and histamine-induced neutrophile and eosinophile adhesion (Advenier et al 1992, O´Donnell and Anderson 1995, Baluk and McDonald 1994, Zink et al 1995). Lastly, eformoterol causes vasodilatation in the pulmonary circuit (Hoffman and Lefkowitz 1996).
We herein report that eformoterol caused a reduction in frequency and severity of endoscopically assessed EIPH in in-training thoroughbred high-performance horses, in a blind trial carried out during standard training sessions in a naturalistic environment.
MATERIALS AND METHODS
We studied 180 training sessions in 29 high-performance horses with endoscopically confirmed EIPH (male and female, 2-4 years-old, 400-500 kg). Animals were on a normal feeding schedule. The first 90 sessions were used to classify horses according to the severity of bleeding. Thoroughbreds were classified -on the basis of the endoscopic assessments prior to the administration of the experimental drug- into two categories:
1- Light bleeders (LB, maximum endoscopic evaluation 2+).
2- Heavy bleeders (HB, endoscopic evaluation greater than 2+ in any training session).
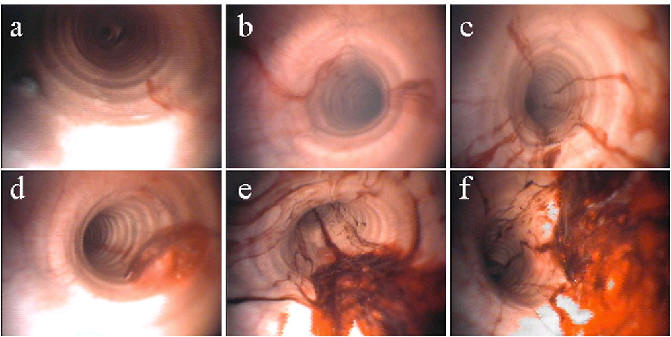
Endoscopic assessments were carried out 60 min after the end of the session, and were classified as shown in Figure 1, except the presence of epistaxis which was assessed by visual inspection.

Figure 1. Endoscopic Assessment of EIPH. Assessments were carried out 60 min after the end of the training session with a 110 cm flexible fiberendoscope without sedation, with a twitch for restraint. This allows detection of visible abnormalities from the nasal passages to the tracheobronchial tree. The presence of blood was read as: drops (a), traces (b), ½ + (c), 1+ (d), 2+ (e), or greater than 2+ (f).
Animals worked in the San Isidro racetrack (Buenos Aires, Argentina), race field #2, between 1/28/2000 and 12/4/2000. During working sessions, field temperature oscillated between 0-25ºC, track conditions were normal to heavy and all speed measurements were taken on the same segment of track. Work sessions consisted of exercises performed at a minimum speed of 15.5 m/sec over 600 to 1800 meters. Eformoterol (Arterol, Laboratorio Fundación, Argentina) was administered intramuscularly in the neck, in a single dose 2 hours before the onset of the training session. Thoroughbreds classified as LB received a dose of 0.04 mg, and those classified as HB received a dose of 0.08 mg. Vital signs were recorded at the time of injection (t0, whether or not eformeterol was given) and 60 minutes after the end of the training session. We measured heart rate (HR), respiratory rate (RR), depth of respiration, heart arrhytmias (auscultation), and arterial pressure (measured in the left front leg at middle radius level with sphigmomanometer and auscultation). To evaluate performance, jockeys and trainers, blind to pharmacological treatment, filled evaluation cards after each training session according to the following qualitative scale:
Outstanding: no complaints or concerns
Very Good: may lack speed or strength at the end of the run
Good: may lack speed or strength during the run (“didn´t run well”)
Fair: may become short of breath, could not finish with speed.
Poor. became short of breath, could not finish the run.
The average speed was very similar in the experimental and control sessions, and was not different between LB (15.57-17.73 m/sec) and HB (15.53-16.99 m/sec). As described, work sessions were similar in intensity to competitive training. The race track is made of sand, and for most days during the trial it was heavy, with cool temperatures (0-25º C).
RESULTS
Administration of eformoterol resulted in a marked decrease in bleeding episodes (Figure 2).
Figure 2. Effect of eformoterol on endoscopic bleeding. Endoscopic assessments of thoroughbred horses during training sessions with (Eformeterol) and without (Control) intramuscular eformoterol. All assessments were performed by a veterinarian blind to the treatment received by the animals. Slightly more than half of the animals were heavy bleeders (HB) at baseline, and received 0.08 mg of eformoterol. The rest of horses entered in the study bled between 1 and 2+ (LB), and received 0.04 mg of eformoterol. Eformoterol resulted in a significant decrease in bleeding severity in LB (c2:89.919, df=5, p = <0.001) and in HB (c2:111.404, df=5, p = <0.001). no: normal endoscopic assessment.
Epistaxis during training with eformoterol was not observed regardless of the previous classification of the horses. All horses classified as LB showed less than 2+ blood under endoscopy after administration of 0.04 mg eformoterol (95% were read as normal or less than 1+). Only one horse classified previously as HB showed endoscopic bleeding greater than 2+ after administration of 0.08 mg eformoterol (85% were between normal and 1+). A summary of the vital signs of the thoroughbreds at the end of the training session is shown in Table 1.
Light bleeders
Heavy bleeders
baseline
40' post-exercise
baseline
40' post-exercise
Heart Rate
35±0.5
46±1.7
38±0.7
45±4.7
Arrythmia
0
0
2
0
Resp rate
14±0.4
21±1.3
16±0.7
19±1.8
Deep breathing
no
yes
no
yes
Blood pressure
158±3.5
175±5
150±4
160±2.2
Table 1. Vital signs of Thoroughbreds suffering from EIPH following eformeterol treatment prior to intense exercise (average speed 16 m/sec ± 0.75). Baseline values were recorded immediately prior to the administration of eformeterol, and 40' post exercise values were recorded upon return of the animals to their box. Values represent mean ± SEM. .
Vital signs returned to baseline within 40 minutes after exercise in eformoterol-treated horses.
DISCUSSION
The defining feature of EIPH is the relation of pulmonary bleeding to intense exercise, which has been observed in a variety of horse breeds (Lekeux et al, 1993; Stephen and Waewick, 1998). EIPH is exacerbated by age and speed, such that its incidence may be as high as 90% in thoroughbreds exercising with speeds greater than 14 m/sec (Sweeney CR, 1991, Lekeux et al, 1993). In our experience, there is a direct correlation between incidence of EIPH and effort magnitude (jockey demands, race track quality, etc), as well as the temperament of the horse. Thus, under routine training conditions there is a great deal of variability in the severity of bleeding. To minimize this type of variability, we standardized daily working conditions and assessed bleeding endoscopically after the animals practiced on a fixed segment of the race track, and with average speeds of 16 m/sec. In addition, we observed each horse on several training sessions with and without treatment and evaluated them blindly. In common veterinary practice, routine endoscopic examination is performed 30 to 120 minutes after exercise (Pascoe et al 1981, Raphel and Soma 1982, etc). We chose 60 min as an intermediate value.
The presence of severe bleeding (greater than 2+) in any of the preliminary training sessions classified the horse as a HB. Administration of eformoterol resulted in a significant decrease in the severity of the bleeding episodes. Performance assessments by the jockeys and trainers, who were blind to the treatment, were "outstanding" in every case, whereas control assessments were on average "good" (data not shown). This could be attributed to a direct effect of the decrease bleeding, but we cannot exclude alternative explanations such as improved ventilation or perfusion. We are currently collecting data to address this question specifically. The only evident side effect of eformoterol was sweatiness at the injection site, likely caused by a direct b2 adrenergic action, since apocrine glands receive this kind of inervation in horses. In summary, we have shown that eformoterol significantly reduces EIPH in thoroughbreds undergoing competitive training. Most likely, the remarkable protective effect of eformoterol is due to a combination of vasodilatation, reduction of microvascular permeability (thus avoiding edema), inhibition of inflammatory factors, and reduction or closing of the endothelial gap (Barnes PJ, 1993; Faulds et al, 1991; Greiff et al, 1998; Lindberg et al. 1995; Mita and Shida, 1983; Nighitingale et al.1999; O’Donnell and Anderson 1995; Persson CGA, 1993). Eformoterol-induced bronchodilation may reduce transmural alveolar pressure and also contribute to the protective effect against EIPH.
REFERENCES
Advenier C, Qian AND, Koune JD, Molimard M, Candenas ML, Naline AND (1992). Formoterol and salbutamol inhibit the permeability microvascular induced by the bradykinin–and histamina in the Guinea pig. Br J Farmacol;105:792.
Anderson GP (1993). Formoterol: pharmacology, molecular basis of agonism, and mechanism of long duration of a highly potent and selective beta 2-adrenoceptor agonist bronchodilator. Life Sci; 52(26):2145-2160.
Baluk P, McDonald DMK (1994). The beta 2-adrenergic receptor agonist formoterol reduces microvascular leakage by inhibiting endothelial gap formation.Am J Physiol 266:L461-8
Barnes PJ (1993) b-receptors on smooth muscle, nerves and inflammatory cells. Life Sci 52 (26):2111-2121.
Birks EK., Mathieu-Costello O, Fu Z, Tyler WS,. West JB (1997). Very high pressures are required to cause stress failure of pulmonary capillaries in Thoroughbred racehorses J. Applied Physiology 82(5)1584-1592
Donaldson LL (1991). A review of the pathophysiology of exercise-induced pulmonary haemorrhage in the equine athlete. Vet Res Commun;15(3):211-26
Faulds D, Hollingshead LM, Goa, KL (1991). Formoterol: a Review of its pharmacological properties and therapeutic potential in reversible obstructive airways disease. Drugs; 42(1):115-137.
Greiff L, Wollmer P, Andersson M, Svensson C, Persson CG (1998). Effects of formoterol on histamine induced plasma exudation in induced sputum from normal subjects.Thorax; 53(12):1010-3
Gunson DE, Sweeney CR, Soma LR. (1988) Sudden death attributable to exercise-induced pulmonary hemorrhage in racehorses: nine cases (1981-1983). J Am Vet Med Assoc; 193(1):102-6
Hoffman BB and Lefkowitz RJ. (1996) Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists. in: Goodman and Gilman's The Pharmacological Basis of Therapeutics, 9th Edition, Section II, chapter 10.
Johnson AT, Soma LR, Ferouz C (1992). Modelling exercise-induced pulmonary hemorrhage in racing thoroughbreds. Front Med Biol Eng; 4(4):271-89
Lapointe JM, Vrins A, McCarvill E (1994). A survey of exercise-induced pulmonary haemorrhage in Quebec standardbred racehorses. Equine Vet J;26(6):482-5
Lekeux P, Clerex C, Art T (1993). Functional effects of vascular pulmonary diseases. in: Pulmonary function in healthy, exercising and diseased animals. Flemish Veterinary Journal, Special issue pp: 267-305
Lindberg S, Khan R, Runer T (1995).The effects of the formoterol in the activity mucociliar. Eur J Farmacol 285 :275.
Mita H, Shida T (1983). Anti-allergic activity of formoterol, a new beta-adrenoceptor stimulant, and salbutamol in human leukocytes and human lung tissue. Allergy 38(8):547-52
Nightingale JA, Rogers DF, Barnes PJ (1999). Differential effect of eformoterol on adenosine monophosphate and histamine reactivity in asthma. Am J Respir Crit Care Med ;159:1786.
O'Callaghan MW, Pascoe JR, Tyler WS, Mason DK (1987). Exercise-induced pulmonary haemorrhage in the horse: results of a detailed clinical, post mortem and imaging study. VIII. Conclusions and implications. Equine Vet J, 19(5):428-34
Ochsner M (1996). Simultaneous measurement of Ca2+ transients and changes in the cell volume and microviscosity of the plasma membrane in smooth muscle cells. Evaluation of the effect of formoterol. Biochem Pharmacol ;52(1):49-63
O'Donnell SR, Anderson GP (1995). Mass balance and metabolism of [(3)H]Formoterol in healthy men after combined i.v. and oral administration-mimicking inhalation.Br J Pharmacol 116:1571.
Oikawa M. (1999). Exercise-induced haemorrhagic lesions in the dorsocaudal extremities of the caudal lobes of the lungs of young thoroughbred horses. J Comp Pathol 121(4):339-47
Pascoe JR, Ferraro GL, Cannon JH, Arthur RN, Wheat JD. (1981) Exercise-induced pulmonary hemorrhage in racing thoroughbreds:a preliminary study. Am J Vet Res 42(5):703-707.
Persson CGA (1993). The action of b-receptors on microvascular endothelium or: Is airways plasma exudation inhibited by b-agonists? Life Sci, 52:2111-2121.
Raphel CF, Soma LR.(1982) Exercise-induced pulmonary hemorrhage in Thoroughbreds after racing and breezing. Am J Vet Res; 43(7):1123-7
Stephen and Waewick (1998) Equine Internal Medicine, 281-283; Saunders Co.
Sweeney CR (1991) Exercise induced pulmonary hemorrhage. Vet Clin North Am Equine Pract.,7:93-104.
Takahashi T, Hiraga A, Ohmura H, Kai M, Jones JH. (2001).Frequency of and risk factors for epistaxis associated with exercise-induced pulmonary hemorrhage in horses: 251,609 race starts (1992-1997). J Am Vet Med Assoc; 218(12):1965
Verleden GM, Belvisi MG, Rabe KF, Miura M, Barnes PJ (1993). Beta 2-adrenoceptor agonists inhibit NANC neural bronchoconstrictor responses in vitro; J Appl Physiol 74(3):1195-9.
Zink S, Cabbage Rosen P, Lemoine H (1995). Regulation of the permeability transendothelial for those beta-adrenergicos in the cells endotheliales of the channel micro and macrovascular. Am J Physiol; 269:1209
©1994-2003 All Rights Reserved. Online Journal of Veterinary Research. You may not store these pages in any form except for your own personal use. All other usage or distribution is illegal under international copyright treaties. Permission to use any of these pages in any other way besides the before mentioned must be gained in writing from the publisher. This article is exclusively copyrighted in its entirety to OJVR publications. This article may be copied once but may not be, reproduced or re-transmitted without the express permission of the editors.